Overview
An energy transformation is underway, moving toward an "electron" rather than fossil fuel energy economy. The constant delivery of clean electrical energy presents a global challenge - once electrical energy is generated, it must be used, or it is wasted. To overcome this challenge, energy storage has a strategic importance to bridge the gap between energy generation and demand, support a transportation revolution toward electrified vehicles, and ensure that the energy transition uplifts all individuals and communities regardless of socioeconomic status. At I:SEE, researchers are building the scientific foundation for advanced energy storage systems that meet current and future energy needs.
Research Area 1 - Scalable Energy Storage
I:SEE researchers are investigating scalable, cost-effective, and environmentally safe energy storage materials and systems to support full integration of renewable forms of energy such as wind and solar into a modernized electric grid. Grid-scale batteries using water as the electrolyte rather than the flammable solvents traditionally used for lithium-ion batteries are under study.
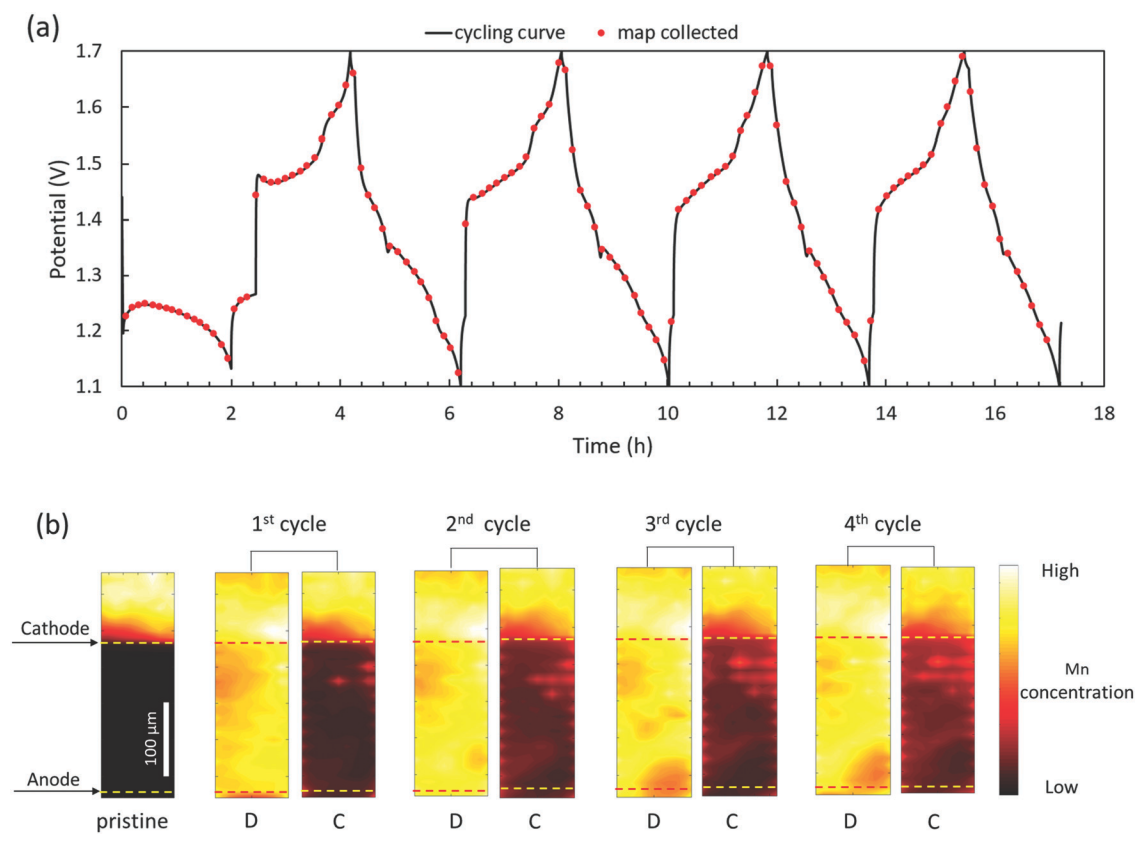
I:SEE scientists and collaborators demonstrated faradaic Mn dissolution and deposition
as the dominant charge transfer reaction in aqueous zinc manganese oxide batteries
via synchrotron-based X-ray fluorescence mapping. [BNL article]
Daren Wu, Lisa M. Housel, Sung Joo Kim, Nahian Sadique, Calvin D. Quilty, Lijun Wu,
Ryan Tappero, Sarah L. Nicholas, Steven Ehrlich, Yimei Zhu, Amy C. Marschilok, Esther
S. Takeuchi, David C. Bock, Kenneth J. Takeuchi, “Quantitative temporally and spatially
resolve X-ray fluorescence and microprobe characterization of the manganese dissolution-deposition
mechanism in aqueous Zn/a-MnO2 batteries,” Energy and Environmental Science, 2020,13, 4322-4333. [DOI: 10.1039/D0EE02168G].
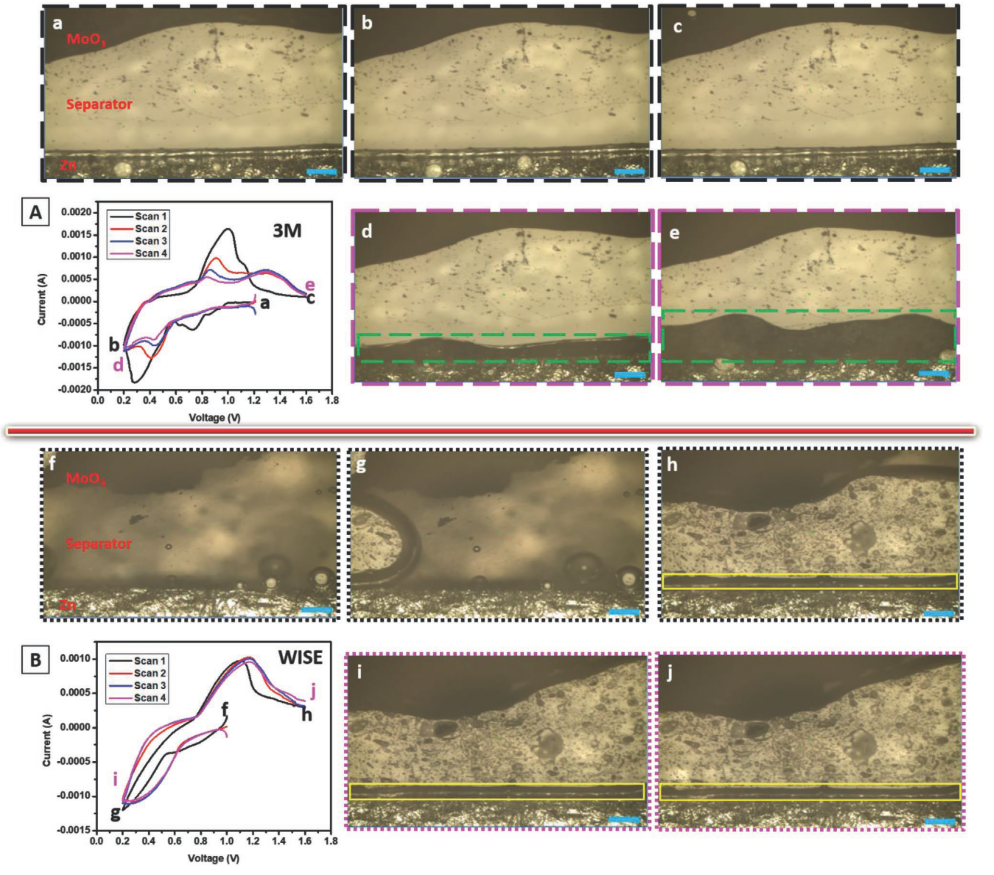
I:SEE researchers report a zinc chloride water-in-salt electrolyte that increases
stability of an aqueous zinc/molybdenum oxide battery by protecting the zinc anode
surface from dissolved molybdenum species and minimizing hydrogen gas evolution.
Lei Wang, Shan Yan, Calvin D. Quilty, Jason Kuang, Mikaela R. Dunkin, Steven N. Ehrlich,
Lu Ma, Kenneth J. Takeuchi, Esther S. Takeuchi, Amy C. Marschilok “Achieving Stable
Molybdenum Oxide Cathodes for Aqueous Zinc-Ion Batteries in Water-in-Salt Electrolyte,”
Advanced Materials Interfaces, 2021,8, 2002080. [DOI: 10.1002/202002080].
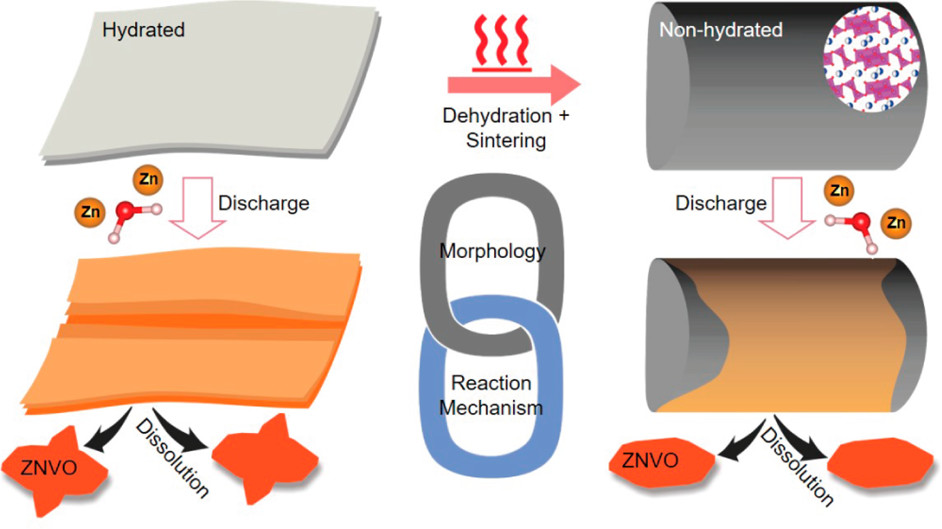
TEM observations reveal hydration level and nanostructure size of sodium vanadate
oxide cathodes significantly impact level of charge storage in zinc-ion aqueous batteries.
Sung Joo Kim, Christopher R. Tang, Gurpreet Singh, Lisa M. Housel, Shinze Yang, Kenneth
J. Takeuchi, Amy C. Marschilok, Esther S. Takeuchi “New Insights into the Reaction
Mechanism of Sodium Vanadate for an Aqueous Zn Ion Battery,” Chemistry of Materials, 2020,32, 2053-2060. [DOI: 10.1021/acs.chemmater.9b05103].
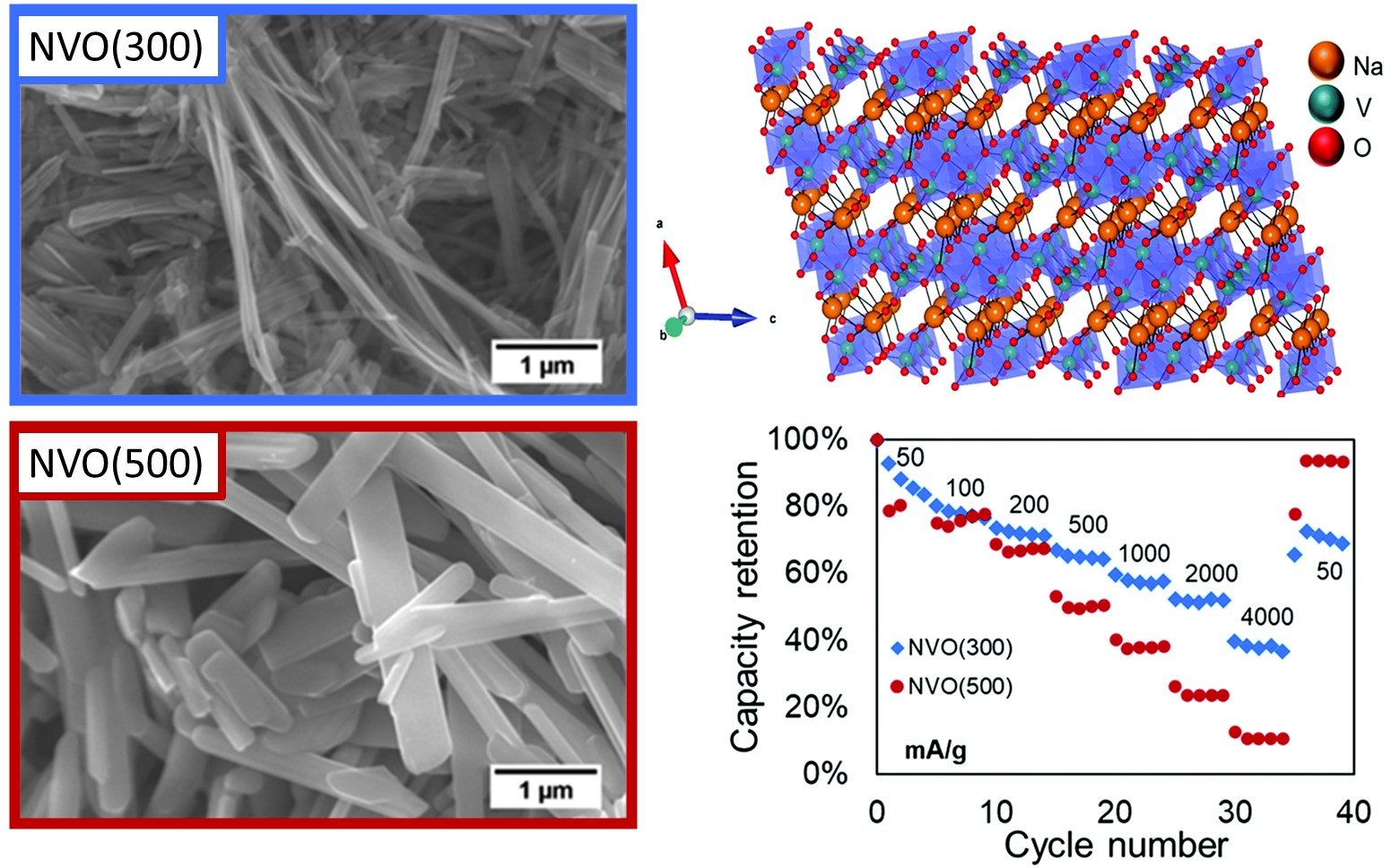
I:SEE results demonstrated that variation of heat treatment of sodium vanadium oxide
materials lead to differences in material properties that affect the aqueous zinc
electrochemistry. The samples had distinct cyrstallite, particle size, interlayer
water, and surface area and impacted the delivered capacity, rate capability, and
capacity retention of zinc-ion aqueous cells. The electrochemical electron transfer
was noted to be linked to change in vanadium oxidation state through the use of operando
X-ray absorption spectroscopy
Christopher R. Tang, Gurpreet Singh, Lisa M. Housel, Sung Joo Kim, Calvin D. Quilty,
Yimei Zhu, Lei Wang , Kenneth J. Takeuchi, Esther S. Takeuchi, Amy C. Marschilok “Impact
of sodium vanadium oxide (NaV3O8, NVO) material synthesis conditions on charge storage
mechanism in Zn-ion aqueous batteries,” Physical Chemistry Chemical Physics, 2021,23, 8607-8617. [DOI: 10.1039/D1CP00516B].
Research Area 2 - Transportation-Relevant Energy Storage
I:SEE researchers are studying reaction mechanisms and improving design of high-energy,
high-power electrode materials and systems to accelerate adoption of electric vehicles.
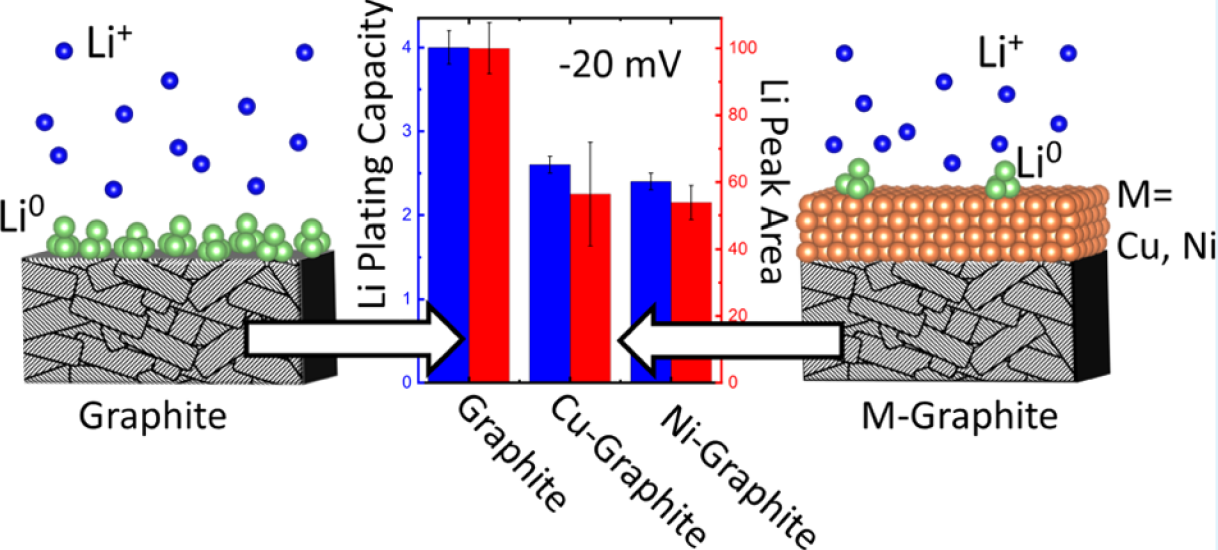
I:SEE researchers demonstrate interfacial modification of graphite electrodes via
direct current (DC) magnetron sputtering of nanoscale layers of Cu and Ni as a means
to increase the overpotential for Li deposition and suppress Li plating under high
rate charge condition. This research may help pave the way toward faster charging
cars.
Killian R. Tallman, Bingjie Zhang, Lei Wang, Shan Yan, Katherine Thompson, Xiao Tong,
Juergen Thieme, Andrew Kiss, Amy C. Marschilok, Kenneth J. Takeuchi, David C. Bock,
Esther S. Takeuchi “Anode Overpotential Control via Interfacial Modification: Inhibition
of Lithium Plating on Graphite Anodes,” ACS Applied Materials & Interfaces, 2019,11, 46864-46874. [DOI: 10.1021/acsami.9b16794].
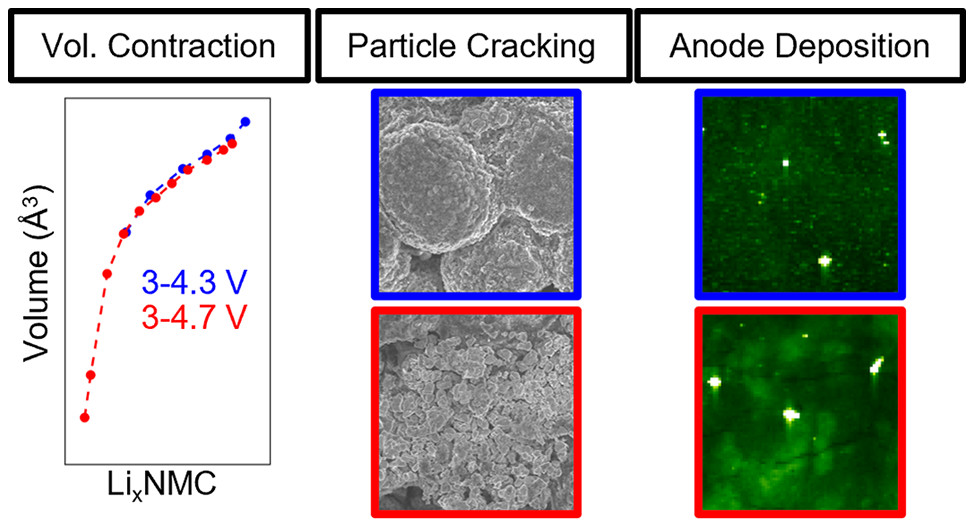
The degradation mechanisms of lithium nickel manganse cobalt oxide, a cathode system
often used for electric vehicles, at high and low voltage are revealed through X-ray
absorption spectroscopy, X-ray fluorescence mapping, diffraction, microscropy, and
electrochemical testing. The results are important toward understanding the trade-offs
of battery use conditions versus their life time.
Calvin D. Quilty, Garrett P. Wheeler, Lei Wang, Alison H. McCarthy, Shan Yan, Killian
R. Tallman, Mikaela R. Dunkin, Xiao Tong, Steven Erlich, Lu Ma, Kenneth J. Takeuchi,
Esther S. Takeuchi, David C. Bock, Amy C. Marschilok “Impact of Charge Voltage on
Factors Influencing Capacity Fade in Layered NMF622: Multimodal X-ray and Electrochemical
Characterization,” ACS Applied Materials & Interfaces, 2021,13, 50920-50935. [DOI: 10.1021/acsami.1c14272].
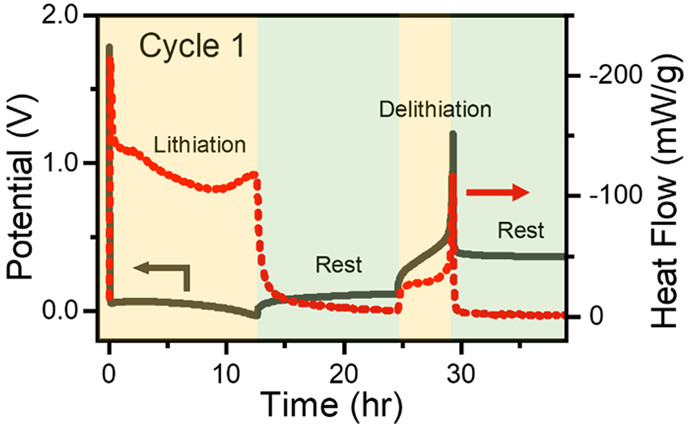
A goal for vehicle batteries is increasing their energy content. One approach is to
use high-capacity silicon as a negative electrode material as that would provide about
10x the capacity of a graphite electrode. The challenge for silicon is the stability
and cycle life of the negative electrodes. I:SEE probes parasitic reactions during
the lithiation of a silicon electrode using operando isothermal microcalorimetry yielding insight into surface-electrolyte interactions
and demonstrating that an approach can be broadly applied to quantify parasitic reactions
in other complex systems.
Lisa M. Housel, Wenzao Li, Calvin D. Quilty, Mallory N. Vila, Lei Wang, Christopher
R. Tang, David C. Bock, Qiyuan Wu, Xiao Tong, Ashley R. Head, Kenneth J. Takeuchi,
Amy C. Marschilok, Esther S. Takeuchi “Insights into Reactivity of Silicon Negative
Electrodes: Analysis Using Isothermal Microcalorimetry,” PACS Applied Materials & Interfaces, 2019,11, 37567-37577. [DOI: 10.1021/acsami.9b10772].
Research Area 3 - Research in Service of Human Health
I:SEE researchers are committed to advancing small, adaptable, and reliable energy
storage systems and characterization approaches that aid the treatment and diagnosis
of human health.
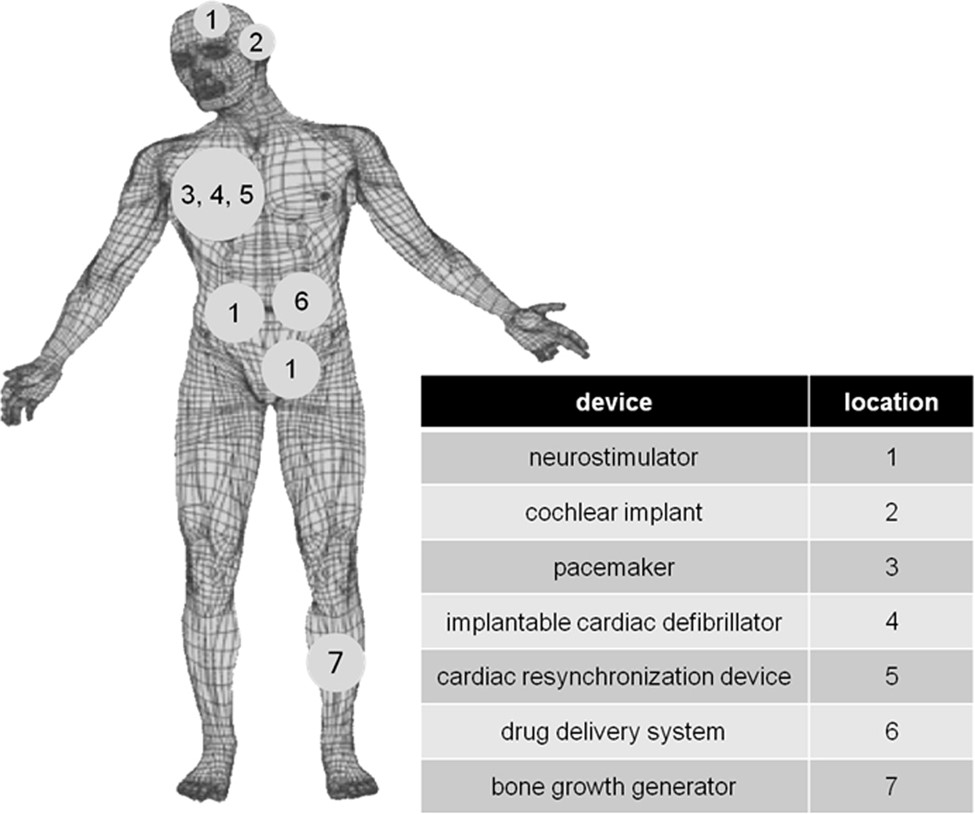
Distinguished Prof. Esther Takeuchi is well-known for inventing and refining the lifesaving
lithium/silver vanadium oxide (Li/SVO) battery technology used in implantable cardioverter
defibrilators (ICDs). I:SEE leverages this deep-rooted expertise to develop new battery
chemistries and address failure modes for pressing biomedical applications.
David C. Bock, Amy C. Marschilok, Kenneth J. Takeuchi, Esther S. Takeuchi “Batteries
used to power implantable biomedical devices,” Electrochemica Acta, 2012,84, 155-164. [DOI: 10.1016/j.electacta.2012.03.057].
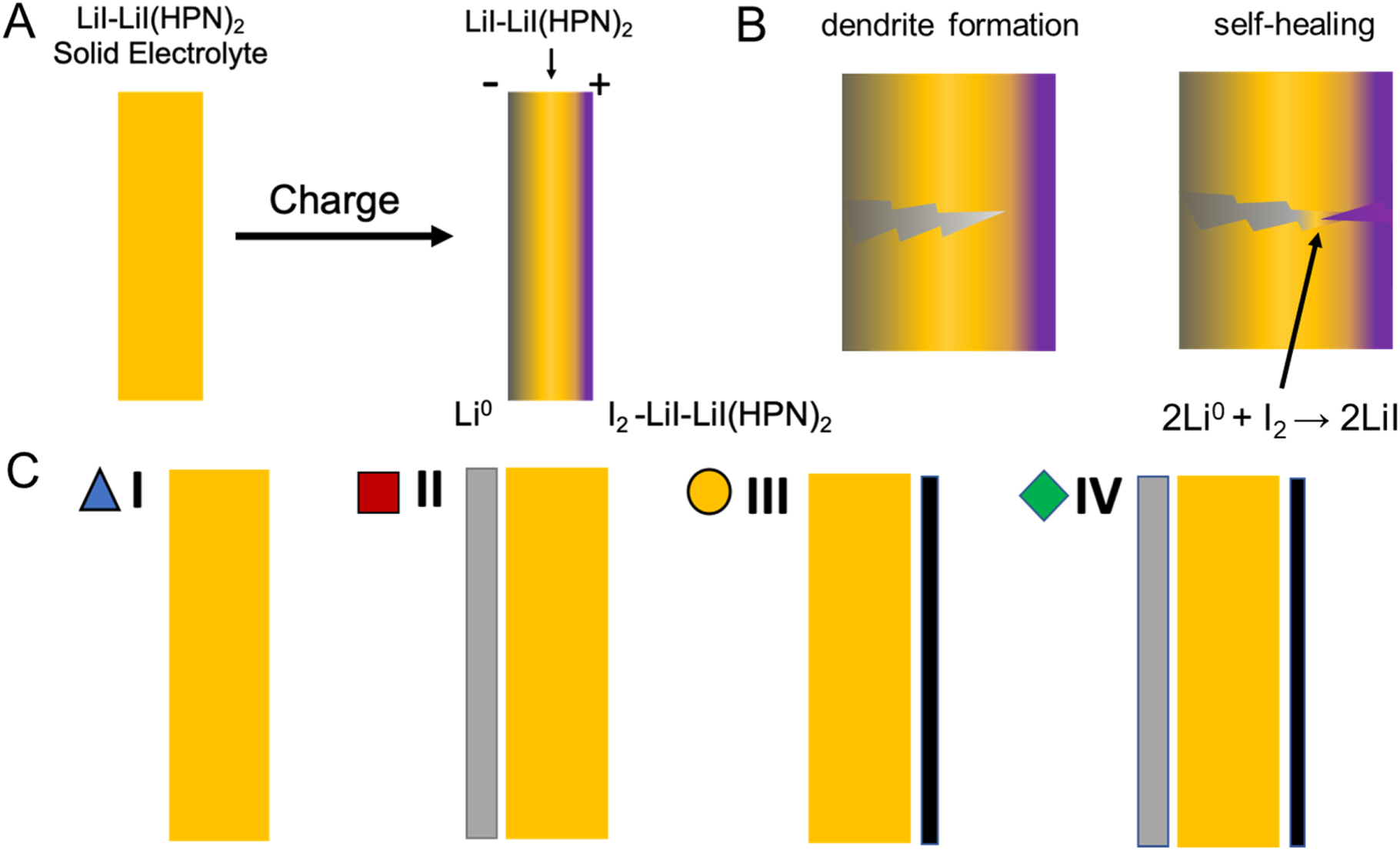
I:SEE scientists are developing a self-forming, self-healing solid-state rechargeable
battery based on the Li/I2 coupling using LiI-rich lithium iodide-(3-hydroxyproprionitrile)2
electrolyte. This battery design builds on the primary Li/I2 batteries, which have
been successfully deployed commercially for implantable pacemakers with an exceptional
record of reliablility. The research demonstrated a self-healing solid-state battery
with high coulombic efficiency.
Chavis A. Stackhouse, Alyson Abraham, Shan Yan, Lei Wang, Nahian Sadique, Gurpreet
Singh, Amy C. Marschilok, Esther S. Takeuchi, Kenneth J. Takeuchi “Self-Healing, Improved
Efficiency Solid State Rechargeable Li/I2 Based Battery,” Journal of The Electrochemical Society, 2021,168 (1), 010519. [DOI: 10.1149/1945-7111/abd831].
I:SEE used synthesis and characterization science developed for energy storage to
understand virus dispersion. Functionalized nanoparticles were synthesized, suspended
in artificial saliva, and dispersed by aerosol onto N95 face masks to mimic coronavirus
transmission. Synchrotron-based X-ray fluorescence mapping effectively visualized
the dispersion of the virus mimic on the masks. This research probed the concept of
adsorption as an important mechanism for effective mask function.
Chavis A. Stackhouse, Shan Yan, Kim Kisslinger, Ryan Tappero, Ashley R. Head, Killian
R. Tallman, Esther S. Takeuchi, David C. Bock, Kenneth J. Takeuchi, Amy C. Marschilok
“Characterization of Materials Used as Face Coverings for Respiratory Protection,”
ACS Applied Materials & Interfaces, 2021,13, 47996-48008. [DOI: 10.1021/acsami.1c11200].